The Centre of Excellence in PK/PD was established to provide complete PK/PD studies. We have extensive experience in experiment design, method development, method validation, quantitative and qualitative sample analysis, statistical analysis, data interpretation, and expert consultation. The Centre of Excellence in PK/PD will deliver the best solutions for your PK/PD study, from beginning through to program completion.
We have an innovative laboratory facility to accommodate various compounds and matrices. Our operators are experienced and understand PK/PD workflows thoroughly, and our experts are always on standby to assist, advise solutions, and review data to check for any errors in real time.
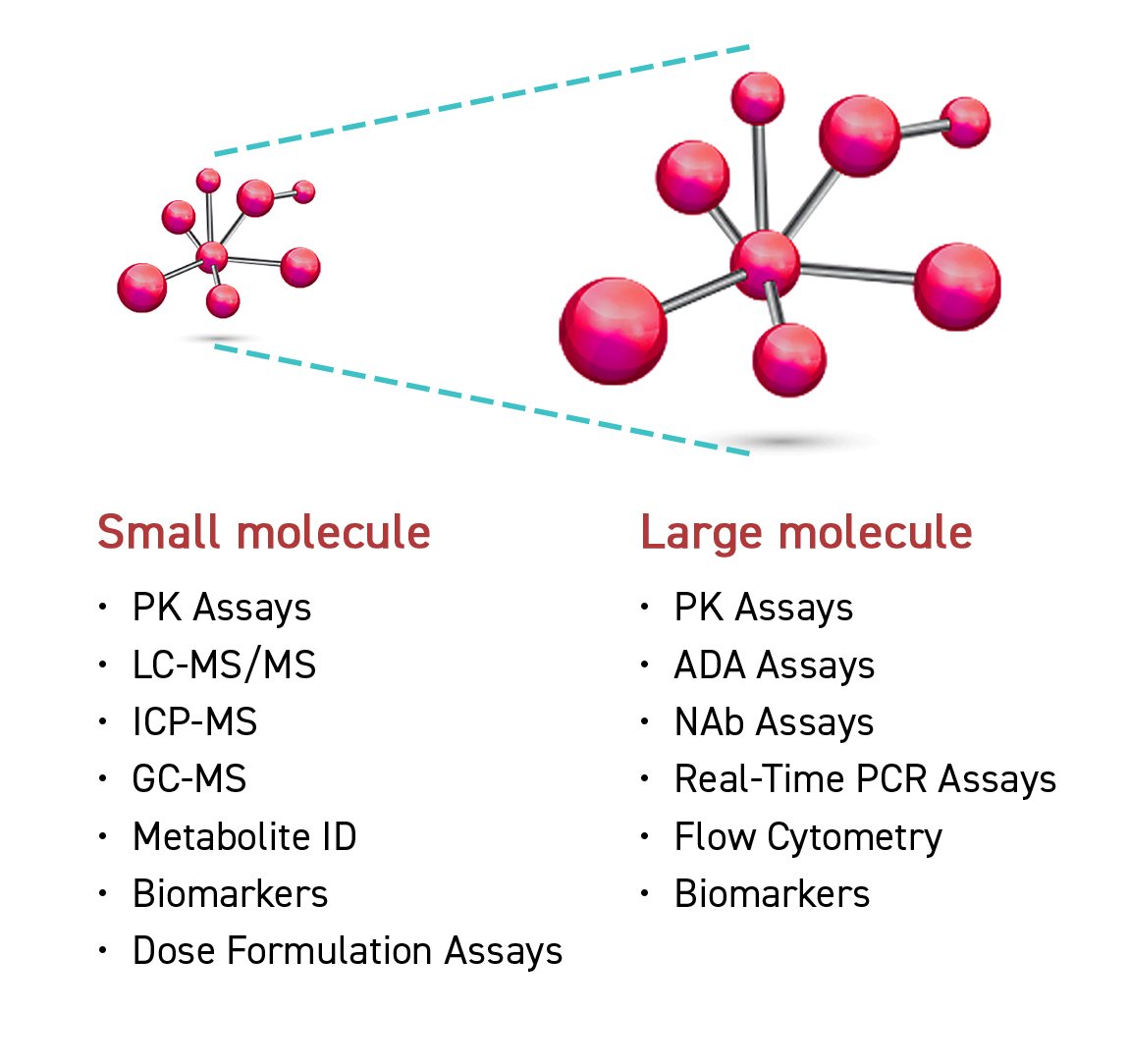
Our analytical method was developed to accommodate a diverse range of biological matrix samples, including plasma, blood, serum, urine, etc. Sample treatment techniques - such as protein precipitation, liquid-liquid extraction, and solid phase extraction - are designed to accommodate a variety of biological matrices as well. Innovative technology is available to perform sample testing, including triple quadrupole liquid chromatography mass spectrometry (QQQ LC-MS/MS) and quadrupole time of flight liquid chromatography mass spectrometry (QTOF LC-MS/MS).
Our LC-MS/MS fully supports analysis for small and large molecules with high sensitivity and resolution. Ultra-high Performance Liquid Chromatography (UHPLC) is an advance chromatography system that can operate under high pressures, help to improve separation efficiency, and shorten run time. An immunogenicity test kit is a quick assay available to provide immunology testing by enzyme-linked immunosorbent assay (ELISA).
At the Centre of Excellence in PK/PD, we are your partner. We work closely with you to ensure the best possible solutions, and power the value of your programs. Our experts provide the scientific and regulatory knowledge to accelerate your drug discovery project to completion.
Services: